African horse sickness (AHS) is an infectious, highly fatal, arthropod-borne disease of horses, mules, and donkeys. There are four recognized types of the AHS, but most of the clinical signs and lesions result from form impairment of the respiratory and circulatory systems. African horse sickness virus (AHSV) is enzootic in most of Africa south of Sahara. It occasionally spreads to other areas with devastating effects on the susceptible equine population. The disease appears to be capable of persisting anywhere in the world when the climatic conditions favor the multiplication of the vector and its survival during the winter months. Epidemics have occurred in the Middle East, Asia and the Mediterranean countries, including in recent years Spain, Portugal, and Morocco. Given the extent of the international movement of horses, AHS could be introduced anywhere in the world, mainly if an animal is imported from an affected country where the disease has not been diagnosed.
What causes African horse sickness?
AHS is caused by a double-stranded, RNA icosahedral virus belonging to the Reoviridae family, genus Orbivirus. It is related to bluetongue and Ibaraki viruses. Nine different serotypes of AHSV has been identified. They can be attenuated for use as a vaccine by the passage in mice and tissue culture.

The virus is destroyed by heating into 70°C for 5 min or 50°C for 10 min by treatment with acetic or citric acid. However, AHS can persist for several months in putrefied blood.
Epidemiology of African Horse Sickness
In endemic areas of Africa, AHS occurs periodically during hot weather following heavy rains. The disease was confined to the African continent until 1944 when it spread to Palestine, Syria, and Jordan. In 1959 there was westward through Turkey to the eastern Mediterranean including Cyprus. The fatality rate among affected animals was nearly 90%, and an estimated total of 300,000 Equidae died of the disease.

The seasonal nature of AHS reflects the activity of the biting midge, Culicoides imicola, which transmits the disease. The midge becomes infected by feeding on a viraemic animal. The virus localizes in the salivary glands and is sent when the midge feeds on susceptible animals. The midge remains infected for life.
What are the symptoms of African Horse Sickness?
Four types of the disease have been described; acute or pulmonary, subacute or cardiac, mixed, and a mild type known as horse sickness fever. The dangerous or pulmonary form is usually seen in susceptible animals during severe epizootics. After a 3-5 day incubation period the horse experiences an acute febrile reaction that may last only 24-48 hr with temperatures as high as 40 degrees C. the fever is followed by respiratory signs associated with pulmonary edema which include tachypnoea, paroxysms of coughing and frothy nasal discharge is voluminous and at the time of death a bubbly liquid may flow from the horse’s mouth. The complete course of the disease is 4-5 days, and the animal drowns in its fluids. The mortality rate is about 90%.
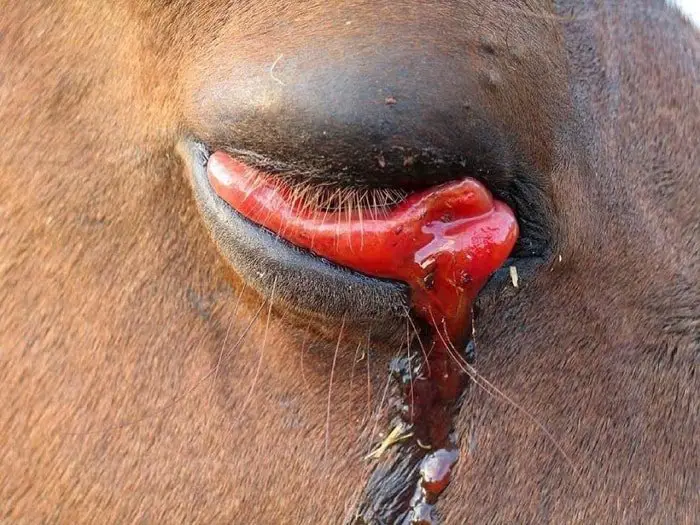
The subacute or cardiac form of the disease is common in enzootic areas. The incubation period lasts 5-10 days and is followed by a febrile reaction for about five days. Pathognomic edematous swellings develop in the head region, especially in the eyelids and supraorbital fossa. The edema frequently extends to the neck and chest but is not gravity dependent. Petechial hemorrhages may develop on the ventral surface of the tongue and the conjunctiva. Abdominal pain often results from bleeding in the gastrointestinal tract. Dyspnoea may be observed, but horses rarely present frothy nasal discharge. The mortality rate is about 50%.
The mixed form of AHS is a combination of the cardiac and pulmonary types. The recent outbreaks in Spain and Portugal were characterized by the mixed model in which either the cardiac or pulmonary form prevailed. Clinical signs were consistent with cardiorespiratory failure with progressive pulmonary edema.

Horse sickness fever is the mildest form of AHS. It is observed in donkeys, zebras, and immunized horses. It presents as a febrile response sometimes accompanied by slight dyspnoea.
Post Mortem Findings of African Horse Sickness
In the majority of the field cases of AHS, the gross lesions are consistent with both cardiac and pulmonary involvement. However, there are specific lessons which characterize each form of the disease. The pulmonary style is characterized by edema of the lungs and hydrothorax. It is possible to find 3-5 L of fluid in the chest cavity. The lungs are hypertrophic with yellowish intestinal foam is almost invariably present in the nostrils. Other lesions may include submucosal congestion of the fundus of the stomach and slight ascites.
The cardiac form is characterized by yellow gelatinous edema of the supraorbital fossa, the intermandibular space and along the fascia of the muscle bundles in the frontal, cervical and sternal regions. There is a marked hydropericardium. The pericardial sac may contain more than 2 L yellowish fluid. Hemorrhages of the epicardium and endocardium are common, and the lungs may be congested. Hydrothorax and ascites are sometimes present. Hyperaemia of the glandular fundus of the stomach, perirenal edema, hyperemia and hemorrhages in the mucosa and serosa of the large and small intestines, subcapsular injury of the spleen and hypertrophy of the mediastinal and mesenteric lymph nodes are also commonly seen.
Diagnosis of AHS
In enzootic areas, familiarity with the disease may permit a presumptive diagnosis on the basis of clinical signs and gross lesions. However, because of the various forms in which the disease appears and the potential to confuse some of the clinical signs and lesions with those of equine infectious anemia, piroplasmosis, purpura haemorrhagia or poisoning, a confirmed laboratory diagnosis is essential.

The virus can be isolated from heparinized blood samples collected from the live animal or tissues collected at post-mortem examination. The spleen is the tissue of choice, but the virus has been isolated from lung, liver, and bone marrow. In the recent outbreak in Spain, AHSV was separated from the bone marrow in the tail of a carcass which had been buried for several weeks. However, to maximize the chances of isolating virus tissue samples should be placed in virus transport medium and kept calm while they are transported as quickly as possible to a virology laboratory.
Isolation of AHSV is achieved by intracerebral inoculation of suckling mice or by injection of monkey kidney cell lines(VERO and MS) or embryonated hen eggs. Virus isolation can be a slow process necessitating several passages in tissue culture or mice. An indirect sandwich ELISA assay that allows the identification of AHSV antigen within a day or two has been developed.
Virus isolation should be performed in parallel with the ELISA as it is necessary to isolate the virus to determine its antigenic type. Isolate is typed by virus neutralization with type-specific antisera.
Clotted blood sample should be submitted to the laboratory for serological examination. Serologic tests for the detection of antibody against AHSV include agar gel immunodiffusion (AGIG), CFT, the SNT, and a recently developed competition ELISA. A present CFT is the most widely recognized internationally. The majority of countries require that Equidae from a country that has experienced an outbreak of AHs test detrimental by CFT prior to importation.
A high CF antibody titer is a good indication of reasonably recent exposure to the virus by natural infection or by vaccination because CF antibodies are not particularly durable and often start to decrease after 6 or 7 wks. SN antibodies take longer to rise, but they more are more durable. Horses with a negligible titer by the CFT are often positive by SNT and ELISA.
Horses which has been exposed to the virus by natural infection mount a more significant and more durable antibody response than those exposed to the virus by vaccination. Approximately 7.57% of horses do not install a detectable antibody response when they receive their first dose of vaccine. However, when a booster is administered, an anamnestic response is elicited, and they usually seroconvert within a week.
Control and Eradication of AHS
Eradication of AHSV in enzootic areas is not feasible as it is impossible to control the vector and the reservoir host has not been identified. However, it has been possible to control and eradicate the virus outside Africa. Stringent management procedures must be implemented to prevent the spread of AHS in susceptible populations requiring the cooperation of all sections of the community.

When the disease is confirmed, there must be a total ban on the movement of Equidae, including the cancellation of all horse shows, sales, race meetings, and other gatherings of Equidae. Strenuous efforts must be made to identify all infected animals and slaughter them. Horse owners should be advised to stable their horses from early evening until morning when the Culicoides are most active and to spray horses and stables with appropriate insect repellents and insecticides. Immediate vaccination of all Equidae and proper identification of vaccinated animals must be undertaken to control an outbreak of AHS. Before the viral serotype is identified, it is necessary to use a polyvalent vaccine, but a monovalent vaccine can be employed when the virus has been typed. A recently developed inactivated vaccine appears to be valid but is more expensive than the traditional live attenuated vaccines.

A control program is essential to verify that the virus has been eradicated from an affected area. The main features of such a program are the regular clinical examination of sentinel horses, the investigation of deaths among Equidae and the monitoring of the Culicoides population.